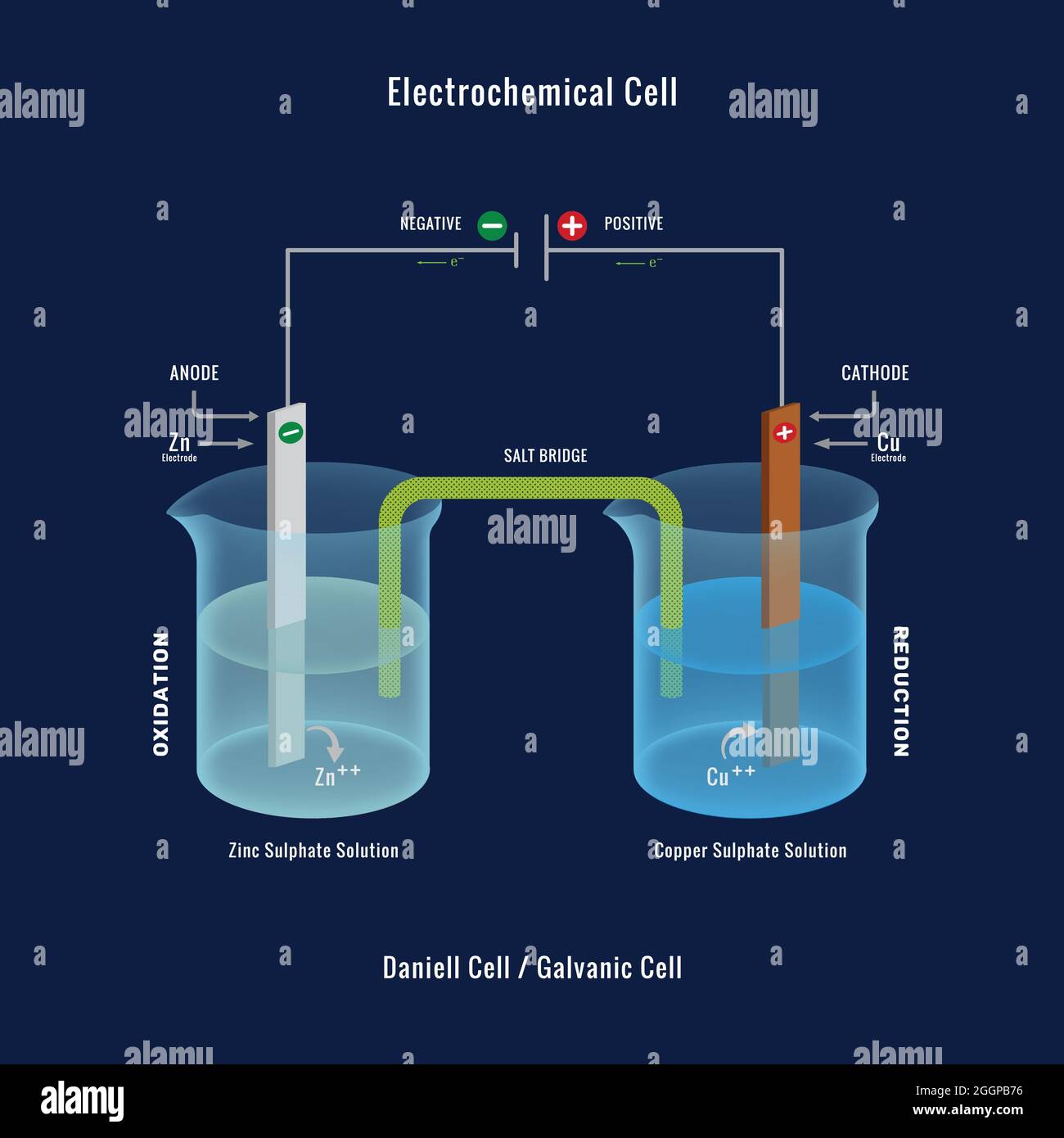
Electrochemical Cell Used For . This kind of cell includes the galvanic,. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. electrochemistry has many common applications in everyday life. components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows. All sorts of batteries, from those used to power a flashlight to a calculator to an. They can also be used on. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind.
from www.alamy.com
They can also be used on. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. components of electrochemical cells. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic,. All sorts of batteries, from those used to power a flashlight to a calculator to an. An electrochemical cell splits the oxidant and reductant in a manner that allows.
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter
Electrochemical Cell Used For electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. electrochemistry has many common applications in everyday life. An electrochemical cell splits the oxidant and reductant in a manner that allows. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. They can also be used on. All sorts of batteries, from those used to power a flashlight to a calculator to an. components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses.
From stoplearn.com
Electrochemical Cells 2023 Electrochemical Cell Used For electrochemistry has many common applications in everyday life. components of electrochemical cells. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. an. Electrochemical Cell Used For.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Electrochemical Cell Used For All sorts of batteries, from those used to power a flashlight to a calculator to an. This kind of cell includes the galvanic,. electrochemistry has many common applications in everyday life. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. An electrochemical cell splits the oxidant and reductant in a. Electrochemical Cell Used For.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Electrochemical Cell Used For All sorts of batteries, from those used to power a flashlight to a calculator to an. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. This kind of cell includes the galvanic,. electrochemistry has many common applications in everyday life. electrochemical cells, also. Electrochemical Cell Used For.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Electrochemical Cell Used For electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. They can also be used on. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. This kind of cell includes the galvanic,. electrochemical cells such as batteries can be used on a. Electrochemical Cell Used For.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Cell Used For an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. They can also be used on. electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic,. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into.. Electrochemical Cell Used For.
From courses.lumenlearning.com
Galvanic Cells Chemistry for Majors Electrochemical Cell Used For electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. This kind of cell includes the galvanic,. components of electrochemical cells. All sorts of batteries, from those used to power a flashlight to a calculator to an. an electrochemical cell is a device that. Electrochemical Cell Used For.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrochemical Cell Used For components of electrochemical cells. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. This kind of cell includes the galvanic,. They can also be. Electrochemical Cell Used For.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Electrochemical Cell Used For All sorts of batteries, from those used to power a flashlight to a calculator to an. An electrochemical cell splits the oxidant and reductant in a manner that allows. This kind of cell includes the galvanic,. electrochemistry has many common applications in everyday life. an apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrochemical Cell Used For.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemical Cell Used For They can also be used on. components of electrochemical cells. electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells, also known as galvanic cells or voltaic cells, are devices. Electrochemical Cell Used For.
From www.dekresearch.com
Shop H Type Electrochemical Cell Sealed 50ml 60ml from Dekresearch Electrochemical Cell Used For They can also be used on. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemistry has many common applications in everyday life. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. An electrochemical cell. Electrochemical Cell Used For.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrochemical Cell Used For electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. An electrochemical cell splits the oxidant and reductant in a manner that allows. All sorts of batteries, from those used to power a flashlight to a calculator to an. This kind of cell includes the galvanic,. They can also be used on.. Electrochemical Cell Used For.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Electrochemical Cell Used For This kind of cell includes the galvanic,. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. All sorts of batteries, from those used to power a flashlight to a calculator to an. An electrochemical cell splits the oxidant and reductant in a manner that allows. components of electrochemical. Electrochemical Cell Used For.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemical Cell Used For an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. All sorts of batteries, from those used to power a flashlight to a calculator to an. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. components of electrochemical cells. This. Electrochemical Cell Used For.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube Electrochemical Cell Used For an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. An electrochemical cell splits the oxidant and reductant in a manner that allows. components of. Electrochemical Cell Used For.
From www.vrogue.co
A Level Aqa Chemistry Questions Electrode Potentials And Cells Revisely Electrochemical Cell Used For electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. They can also be used on. components of electrochemical cells. All sorts of batteries, from those used to power a flashlight to a calculator to an. electrochemistry has many common applications in everyday life. electrochemical cells such as batteries. Electrochemical Cell Used For.
From www.vrogue.co
A Galvanic Cell Is Powered By The Following Redox Rea vrogue.co Electrochemical Cell Used For electrochemical cells such as batteries can be used on a large scale to store energy from intermittent energy sources like solar and wind. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. electrochemistry has many common applications in everyday life. components of electrochemical cells. This kind. Electrochemical Cell Used For.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Electrochemical Cell Used For components of electrochemical cells. electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into. electrochemistry has many common applications in everyday life. This kind of cell includes the galvanic,. They can also be used on. an apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrochemical Cell Used For.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Electrochemical Cell Used For They can also be used on. electrochemistry has many common applications in everyday life. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows. This kind of cell includes the galvanic,.. Electrochemical Cell Used For.